Texas Gov. Greg Abbott announced last Wednesday the Centers for Disease Control and Prevention has made an initial allocation of more than 1.4 million doses of the COVID-19 vaccines for this month.
According to the news release, the vaccines will arrive the week of Dec. 14 and will be distributed to qualifying providers across Texas, who in turn will administer the immunizations based on the Vaccine Distribution Principles developed by the state’s COVID-19 Expert Vaccine Allocation Panel.
Abbott also states that additional allocations may be made later this month and increased amounts are expected in January and the following months.
Initially, the state will allocate COVID-19 vaccines to health care workers, frontline workers, vulnerable populations, areas with health inequities, data-driven allocations and geographic diversity.
The list was made by the panel, which is made up of doctors from the Texas Department of State Health Services, state senators, state representatives and health experts from Texas A&M University and University of Texas System.
One of the members of the panel is Sen. Eddie Lucio, Jr. who represents District 27, which includes counties in the Rio Grande Valley.
Vaquero Radio contacted Lucio for an interview, but he was unavailable as of this broadcast.
Regional Medical Director for the Texas Department of State Health Services Dr. Emilie Prot overlooks Public Health Region 11, which is one of the eight public health regions and includes the RGV.
Prot explains the two pharmaceutical companies that have announced their COVID-19 vaccine that are 95% effective against the virus.
“So, the first one is Moderna and this is a mRNA vaccine,” she said. “It is approved for 18 years and older, and this vaccine does need two doses and they need to be spaced about a month apart or 28 days and this vaccine needs to be frozen or refrigerated. So, their vaccine trial, kind of going back to how many participants have been enrolled is around 30,000 participants … [and] they have reported around 95% efficacy. And then they’ve also reported that this has been a diverse population that have been enrolled in their trial.”
Prot goes into further detail to explain the second pharmaceutical company, Pfizer, that has also been working on the COVID-19 vaccine.
“Then Pfizer, their vaccine trial, they have over 43,000 participants and they have 42% of all of their participants have a diverse background,” she said. “So, this is also [a] mRNA vaccine and it’s approved for ages 12 and over, so a little bit younger than the Moderna. It also requires two doses, and they need to be spaced out at least 21 days. So, both of them around a month [and] the difference with Pfizer is that it needs to be ultra-cold frozen and it can stay five days in a refrigerated or in refrigeration.”
According to Prot, the Pfizer COVID-19 vaccine is also 95% effective.
Prot said having the Pfizer vaccine at ultra-cold frozen presents problems for handling and distribution of the vaccine to communities or populations.
“So, the way it shipped is sort of in these little, it looks like a pizza box type package where it has multiple vials in there, and it can’t be taken out of the ultra-cold of that very cold environment only unless it’s going to be used within those five days,” she said. “So, in our areas that have very low population or those who are pretty remote, it might be a little bit more difficult, but we’re still in the planning stages for that. The state is going to be looking into contracting with different providers. So, there’s already been a call out to many providers, physicians, offices, hospitals, through our immunization program link.”
Dean of UTRGV School of Medicine and Executive Vice President for Health Affairs, Dr. John Krouse, said the university will receive 2,000 Pfizer vaccines.
“We are an identified site for the receipt and distribution of the vaccine, and we should be rolling out to the first identified group sometime this month,” Krouse said.
He said the university will follow the federal and state guidance on prioritizing health care on the first vaccines the university will receive.
“So, what we are going to be doing is immunizing our healthcare faculty and students in this first round later this month, and we’re going to be doing that on our Edinburg campus,” Krouse said. “Actually, we’re setting up an operation in order to give the vaccine at the medical school itself. And we will be doing that at the time that the emergency authorization is finally approved by the FDA sometime this month.”
He also said the university is planning to offer the COVID-19 vaccine the way UTRGV offered the flu vaccine.
“As you know, the vaccine is being produced on a regular basis and only a certain number of doses are available at any one time,” Krouse said. “But we expect in shortly after the first of the year to be receiving a regular supply of vaccine doses that we will target to not only the campus community and certainly we do want to make sure that our campus is safe and that that faculty, staff, and students can return to campus, but certainly we will be having responsibility for the general community in the Valley as well. So, we see this as a process that’s going to continue for at least the next eight or nine months at a minimum.”
According to Prot, evidence shows taking the vaccine will keep people out of the hospital.
“There has been a very few number of people who have, and we’re still looking to get more research on this, but they have gotten either a mild or moderate case of COVID, but the majority … have not ended up in the hospital and all of them have not died,” Prot said. “I think that with all of these vaccinations, there’s not one over the other that has been better.”
She said there is still a lot of unknown such as the length of the protection from COVID-19.
“Some of the studies have shown that most of these vaccines once given they elicit a good immune response and it might depend on the participant [on] which ones have,” Prot said.
According to Pfizer’s website, the company submitted a request to the U.S. Food and Drug Administration for an Emergency Use Authorization of their COVID-19 vaccine candidate.
Prot explains the process of approval for Pfizer’s COVID-19 vaccine.
“So, right now, they’re applying for the EUA or they applied for the EUA, which is the emergency use authorization,” she said. “Then after that, they go to the FDA, the food and drug administration to get, so FDA weighs in on the vaccine and … so they review all of safety data, the advocacy data, to make sure that it is safe and effective because that’s what their role is, is to provide effective and … safe vaccines to the community.”
According to Prot, if the FDA approves the vaccine, the company will be ready to distribute around 6 million doses this month and probably 1 billion in 2021.
Prot explains the vaccine will be free but charges may be made to some.
“So, if it is distributed from a local health department, so people can charge an administration fee and that’s dependent on whoever receives the vaccine, they cannot charge for the vaccine because it’s provided by the feds slash the state,” she said. “So, they can’t charge for the actual vaccine, but they can charge an administration fee. … Then those who receive it through public health, will most likely be free. So, we usually ask for a $10 administration, but then we’re also, you know, if someone can’t pay, we’re not going to turn them back.”
People have shared skepticism on social media of the vaccine and claim they would not want to take it, but former presidents Barack Obama, George W. Bush and Bill Clinton announced they are willing to take the COVID-19 vaccine to promote public confidence.
Krouse said he encourages everyone to get vaccinated when the time comes.
“We really want to encourage people to feel comfortable with their recommendations of both the federal and state governments about the safety and effectiveness of the vaccine,” he said.
Exercise science senior Andy Garcia said he will be getting the vaccine ones it is available to get.
“I just trust the science behind and the technology behind the pharmacy companies developing the vaccine, like Pfizer and Moderna,” Garcia said.
He believes health care workers should be the first ones to get the vaccine.
“They’re the ones who are taking care of people and especially people who are older,” Garcia said. “The CDC recommended for them to get them first, the frontline workers and the nursing homes, and it’s really important that they do because they’re most vulnerable. They’re facing the virus face-to-face; they need to get it as soon as possible.”
According to a news release from Nov. 13, Sunshine Retirement Living is among the senior living companies to enroll in the CDC’s national COVID-19 vaccine program.
The news release states the CDC partnered with CVS and Walgreens to provide free-of-charge, on-site vaccination clinics at senior living communities as soon as a COVID-19 vaccine is approved and becomes available.
Sunshine Retirement Living has partnered with CVS and will offer each of its residents and staff members at its 34 communities across the country the option to receive the free vaccine at the community, including Brook Ridge Retirement Community in Pharr.
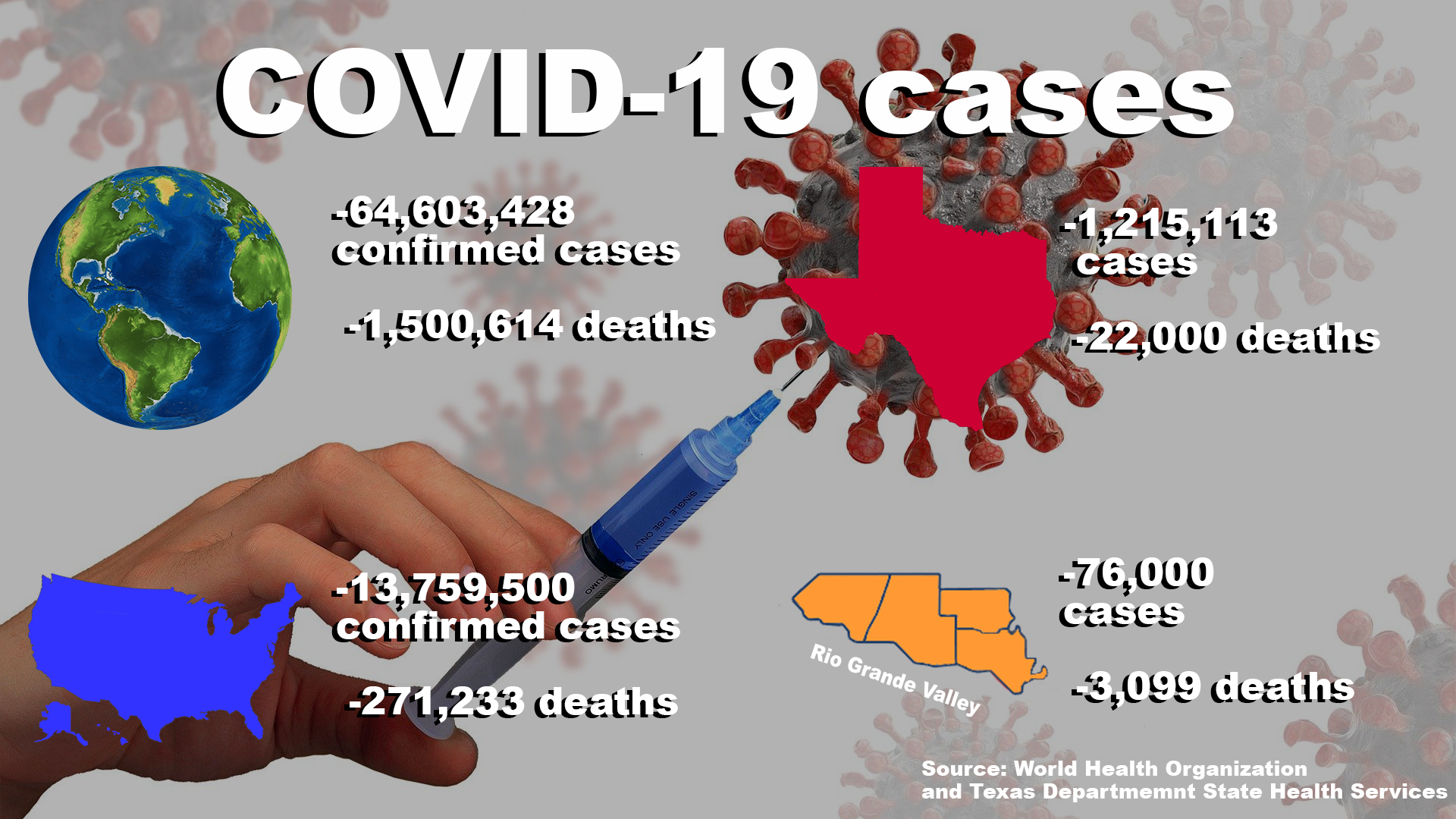
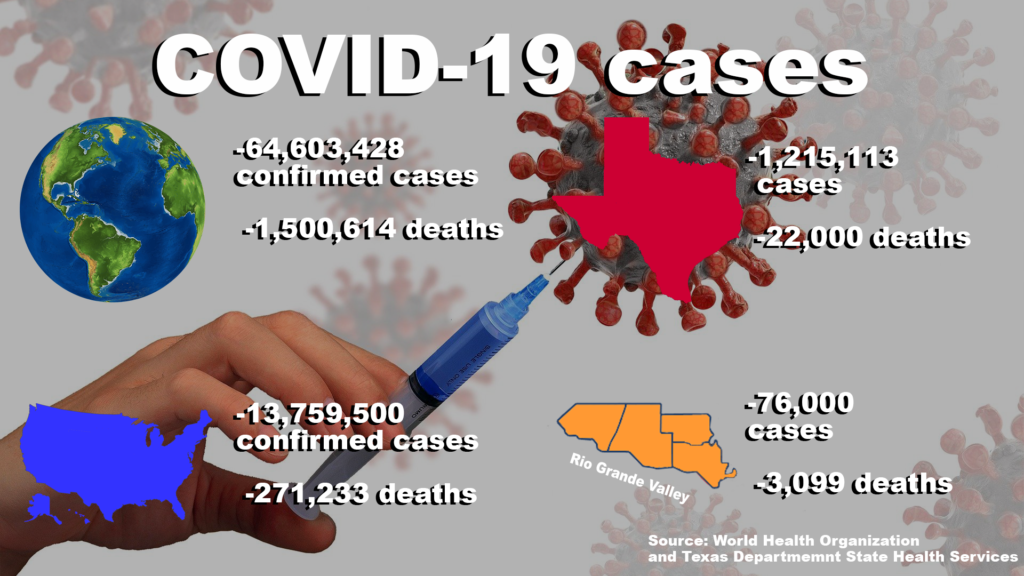
On March 11, the World Health Organization declared the COVID-19 outbreak a global pandemic and as of last Friday, there are currently more than 64,600,400 confirmed cases and more than 1,500,600 deaths.
According to WHO, the U.S. is reporting more than 13,750,500 cases of COVID-19 and more than 270,200 deaths.
As of last Friday, TDSHS has reported more than 1,200,100 cases and 22,000 deaths.
Prot said she encourages Valley residents to continue social distancing and wearing masks.
“Because right now, we still don’t have a vaccine for everyone, but the vaccine news has been very encouraging and very positive,” she said. “So, we’ll see a lot more and expect to hear a lot more from vaccines to vaccination and the next coming weeks and months.”
For Vaquero News, I’m Victor Ramirez.



